The Prime Minister has ordered a review of public health scandals involving the pregnancy test drug Primodos, the use of vaginal mesh implants and the anti-epilepsy drug sodium valproate.
Former Conservative health minister Baroness Cumberlege will lead an examination of the circumstances in all three cases and consider whether there are grounds for wider inquiries into the failings alleged by campaigners.
Sky News last year exposed a cover-up of the impacts of Primodos, a German drug prescribed as a pregnancy test that campaigners say was responsible for causing miscarriages and abnormalities including babies born with missing limbs, brain damage and heart defects.
Research by the manufacturers acknowledged there was a one-in-five chance of birth defects, but the evidence was concealed to frustrate claimants.
Sky News has also been reporting on the impact of vaginal mesh implants for more than two years, drawing attention to women left in acute pain by the procedure, used to treat incontinence and prolapse since the 1990s.

Thousands of women are suffering complications from a "quick-fix" routine operation to cure stress urinary incontinence.
Women have been left in chronic pain, some unable to walk, work or have sex.
The official complication rate set by the medical regulator the MHRA is 1-3%, but Sky News revealed how NHS data shows the figure to be almost 10%.
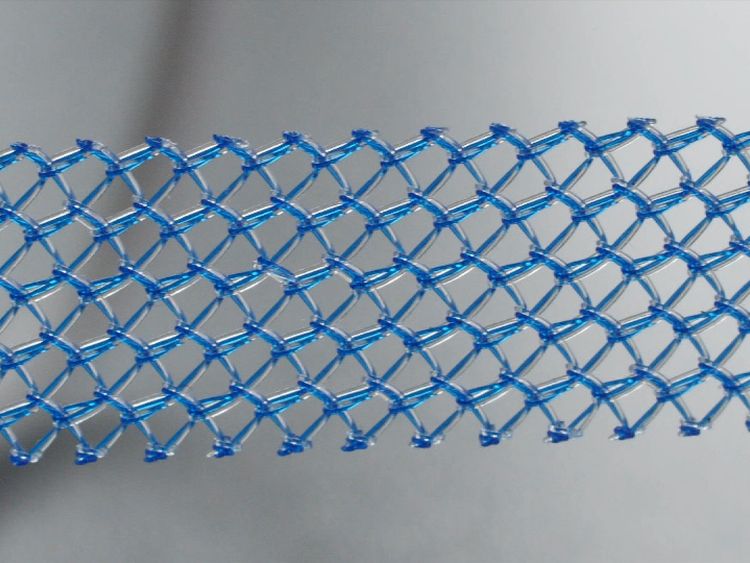
The mesh is made out of polypropylene plastic and designed to be permanent so removing it is a highly dangerous operation.
Last year the use of mesh was heavily restricted in Australia and banned in New Zealand, and the National Institute for Health and Care Excellence has recommended its use be suspended in the UK.
Sodium valproate is an anti-epilepsy drug that has also been linked to birth defects in cases where it was prescribed to expectant mothers without a warning as to the potential consequences.
Documents uncovered by Sky News from the National Archive last year revealed that the dangers to pregnant women were kept from patients for decades despite evidence it could harm foetuses.
More from Primodos

In all three cases, campaigners believe the Government response has been inadequate and have called for full inquiries.
Kath Sampson, from campaign group Sling the Mesh, said she was "thrilled" the review had been ordered but added that "many women's and families' lives have been shattered by medical devices and drugs that were never tested on humans before being released en masse to women globally".
[contf] [contfnew] 
Sky News
[contfnewc] [contfnewc]






